This information is intended for U.S.
healthcare professionals only
Contact: clinicaltrials@argenx.com
This information is intended for U.S.
healthcare professionals only
For More Information,
Contact: clinicaltrials@argenx.com
Contact: clinicaltrials@argenx.com
A Clinical Study for Adults with Ocular Myasthenia Gravis (oMG)
If you have adult patients who are at least 18 years of age with ocular myasthenia gravis, they may qualify to enroll in ADAPT OCULUS, a clinical study to assess an investigational study drug.
For More Information, Contact: clinicaltrials@argenx.com
About ADAPT OCULUS
Who can join?
Consider the ADAPT OCULUS clinical study for your adult patients with myasthenia gravis who are experiencing eye symptoms.
Trial Size
We are recruiting approximately 124 adult participants aged 18 years or older for ADAPT OCULUS, a randomized, double-blinded, placebo-controlled, phase 3, parallel-group design study evaluating the efficacy and safety of efgartigimod rHUPH20 (PH20) Subcutaneous (SC) administered by prefilled syringe.
Study Drug
Efgartigimod PH20 SC is being evaluated for autoimmune diseases mediated by pathogenic IgG autoantibodies, including ocular myasthenia gravis. In this study, the investigational study drug is delivered using a pre-filled syringe. Efgartigimod PH20 SC is not approved by the FDA for the treatment of patients with MGFA class I ocular MG as efficacy and safety have not been established.
See if your patients can be part of this research.
For More Information, Contact: clinicaltrials@argenx.com
KEY Eligibility Criteria
- Patient is an adult
- Patient is not pregnant or planning to become pregnant.
- Patient is MGFA Class I: Any ocular muscle weakness. May have weakness of eye closure. Strength in all other facial, bulbar, and limb muscles is normal. (It is recognized that some patients report fatigue when strength testing is normal. The investigator should use clinical judgment in attributing fatigue to gMG in the absence of objective nonocular weakness.)
- Patient has been diagnosed with Myasthenia Gravis (MG) with consistent clinical features and confirmed by documentation and supported by:
- Seropositivity for AChR-Ab, OR
- Abnormal neuromuscular transmission demonstrated by SFEMG or RNS of ocular or non-ocular muscles (historical or during screening, if needed)
AND
- History of positive edrophonium chloride test, as evidenced by improvement in ptosis or diplopia, or demonstrated improvement in MG signs with treatment such as oral AChE inhibitors, PLEX, IVIg, or corticosteroids.
- Has a screening and baseline MGII (PRO) ocular score of ≥6 with at least 2 ocular items with a score of ≥2.
- Is receiving a stable dosage of MG therapy before screening that includes AChE inhibitors, steroids, or NSISTs, either in combination or alone, with the following dosage conditions:
- Nonsteroidal immunosuppressive drugs (e.g., azathioprine, methotrexate, cyclosporine, tacrolimus, mycophenolate mofetil, and cyclophosphamide) initiated at least 6 months before screening with no change in dosage during the 3 months before screening.
- Steroids initiated at least 3 months before screening, with no change in dosage during the month before screening.
- AChE inhibitors with no change in dosage during the 2 weeks before screening.
- Has symptom onset <3 years ago, unless evidence of MRI without fatty replacement in extraocular muscles or demonstrated response (i.e., improvement in at least 1 oMG sign, based on investigator judgment) to treatment (IVIg, PLEX, pyridostigmine, and/or steroids) in the past year.
- Has no pupillary abnormality other than that from previous local disease or surgery.
- Has no other diseases that lead to eyelid drooping, peripheral muscle weakness, or diplopia.
See if your patients can be a part of this important study
This may be an opportunity for your patients to help in the research for this disease.
If you are interested to see which sites are actively recruiting, please click for an overview of our international study sites. Info is updated monthly at clinicaltrials.gov.
The ADAPT OCULUS study will be conducted at sites across the United States
| Site | Location |
|---|---|
| Neurology Offices of South Florida |
9970 Central Park Blvd N., Ste. 207 Boca Raton, FL 33428 |
| University of South Florida | 13330 USF Laurel Drive Tampa, FL 33612 |
| National Neuromuscular Research Institute |
4705 Spicewood Springs Road, Ste. 200 Austin, TX 78759 |
| Medsol Clinical Research Center | 4161 Tamiami Trl Ste. 120 Port Charlotte, FL 33952 |
| SFM Clinical Research, LLC | 1601 Clint Moore Rd., Ste. 120 Boca Raton, FL 33487 |
| University of North Carolina at Chapel Hill | 101 Manning Dr Chapel Hill, NC 27514 |
| University of Kansas Medical Center Research Institute, Inc. | 4300 Shawnee Mission Pkwy., Ste. 3340 Fairway, KS 66205 |
| Baycare Medical Group | 1201 5th Ave N Saint Petersburg, FL 33705 |
| Neurology Associates PA | 331 N Maitland Ave Dept A-1 Maitland, FL 32751 |
| Duke Early Phase Clinical Research Unit | 40 Duke Medicine Cir Durham, NC 27710 |
For More Information, Contact: clinicaltrials@argenx.com
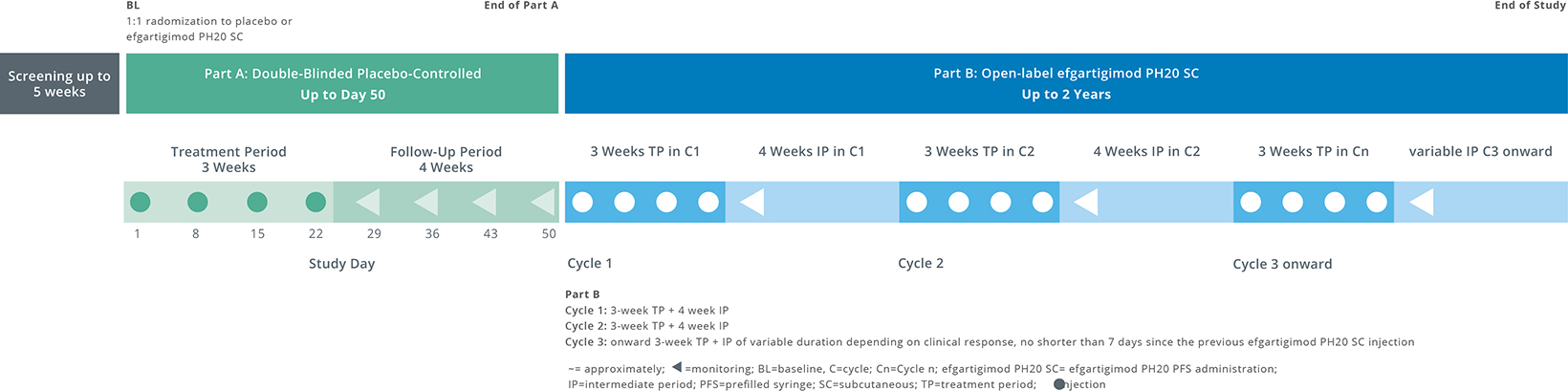
Study Design
ADAPT OCULUS is a clinical study specifically designed for adult patients with oMG. Patients will be asked to participate in the study for more than two years. This will include a screening period of up to five weeks and a two-part study structure with Part A lasting 7 weeks and Part B lasting up to 2 years. In Part A, half of the participants will receive the investigational study drug while the other half will receive a placebo. All participants in the study will receive the investigational drug in Part B of the study.
©2024 Acurian, Inc. All rights reserved.
*In a research study, the participants may receive investigational study product or may receive an inactive substance, or placebo, depending on the study design. Participants receive study-related care from a doctor/research team for the duration of the study. For studies that offer compensation, reasonable payments will be made for participation. The length of the study may vary.
©2024 Acurian, Inc. All rights reserved.